Welcome back to Healthy Innovations! 👋
Earlier this year I did a deep dive on the latest approaches to combating antimicrobial resistance in “Fighting superbugs: The race against antibiotic resistance.”
This week, I'm going deeper - exploring how CRISPR technology is transforming bacteriophage innovation.
Let’s dive in!
Since 1990, antimicrobial resistance (AMR) has killed over one million people annually. In the United States, more than 2.8 million antibiotic-resistant infections occur each year, leading to over 35,000 deaths.
The WHO identifies antimicrobial resistance as one of the top global public health threats. Without new approaches, AMR deaths could rise 67% to 1.91 million annually by 2050 - threatening to unravel a century of medical progress.
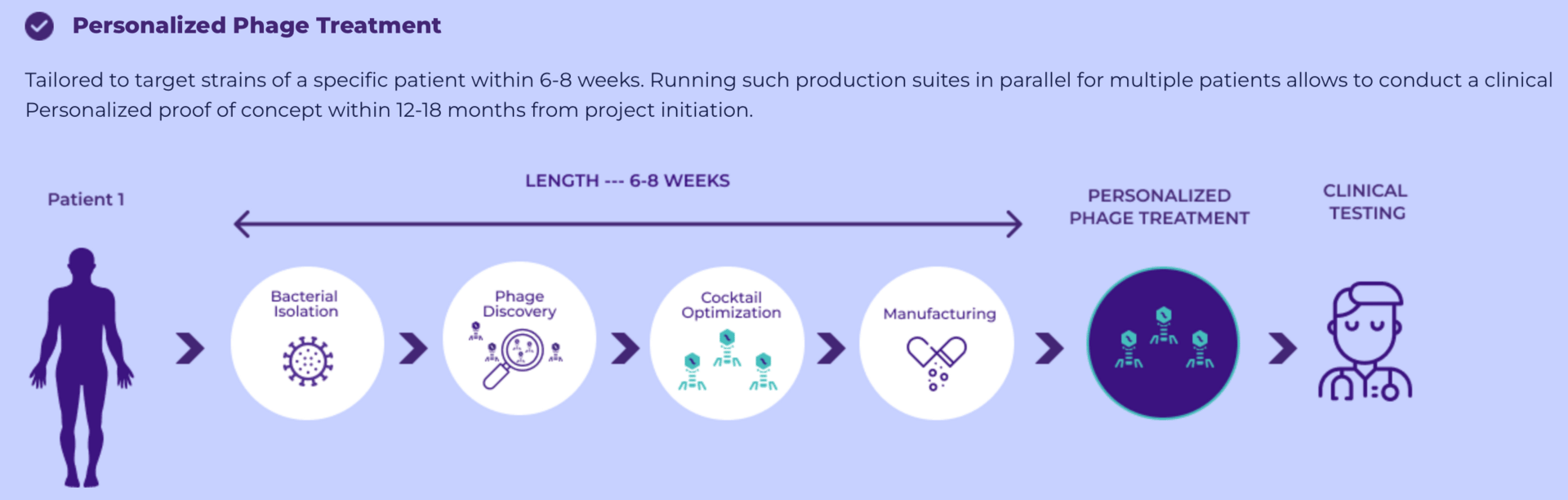
To combat this, scientists are arming nature's oldest bacterial predators with our newest gene-editing tools. These precision-guided viruses hunt down drug-resistant bacteria with remarkable accuracy while leaving beneficial microbes untouched.
CRISPR-enhanced phage therapy marks a shift from broad-spectrum antibiotics to targeted bacterial elimination. Early human trials show these engineered viruses safely reduce resistant bacteria. Two lead candidates are now advancing through clinical development for life-threatening infections.
When antibiotics met their match
Traditional antibiotics work systemically, disrupting the entire microbiome and sometimes triggering complications like C. difficile infections. Worse, resistant bacteria share their resistance genes with neighbors, spreading immunity across species.
The tools we've relied on for nearly a century are failing.
Bacteriophages offer a different approach. These viruses naturally infect and destroy bacteria, outnumbering them 10 to 1 across the planet. When a phage encounters its target bacterium, it injects its DNA, hijacks the cell's machinery, and produces hundreds of new phages that burst out to continue the cycle.
Eastern European doctors used phages to treat bacterial infections long before antibiotics existed. But when penicillin arrived in the 1940s, phage research mostly vanished from Western medicine.
Until now.
Engineering precision at the genetic level
CRISPR technology supercharges natural phages.
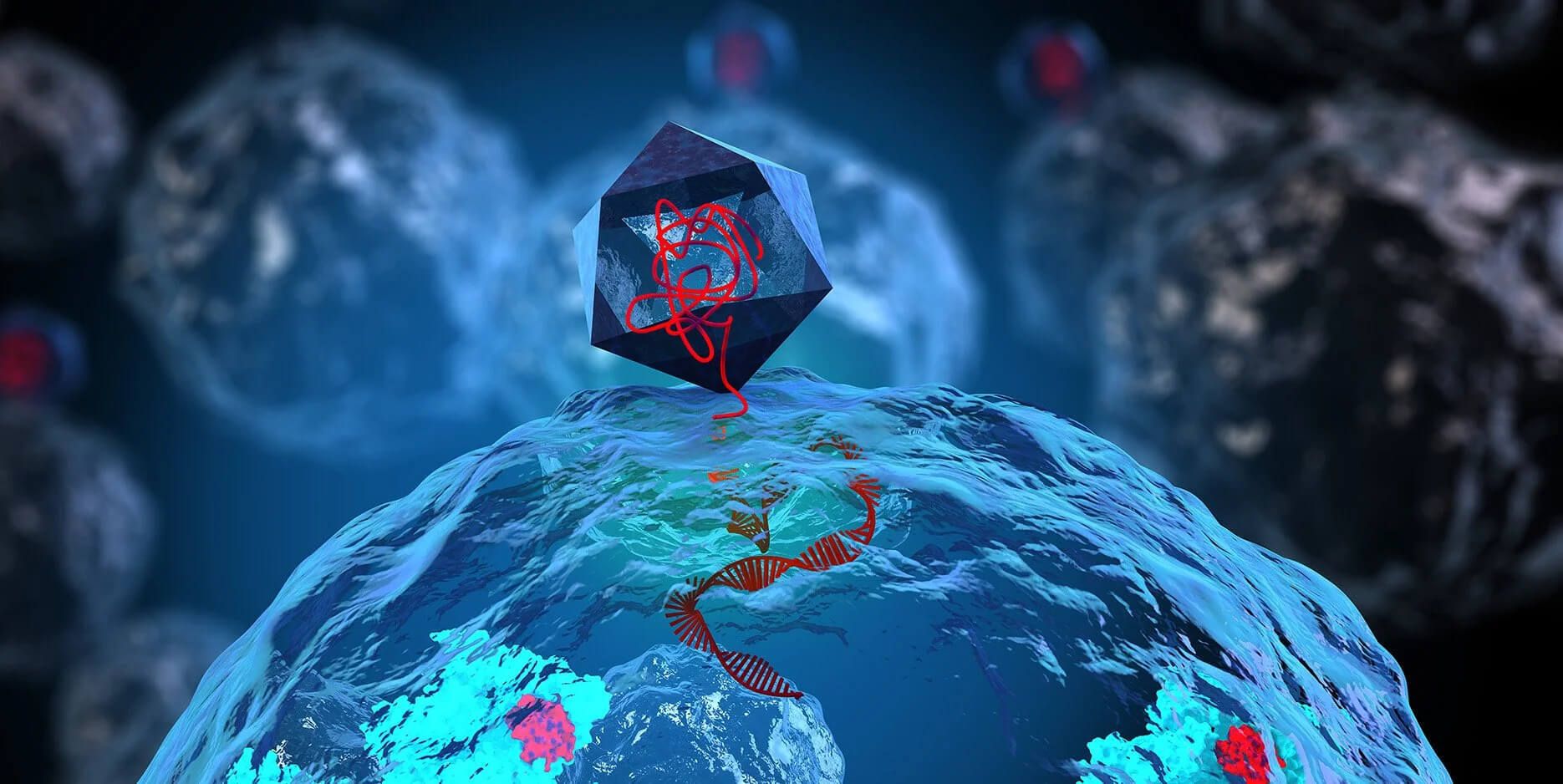
The engineered versions combine the phage's natural ability to infect and replicate within bacteria with CRISPR-Cas systems that slice apart specific DNA sequences in the bacterial genome. These molecular scissors target conserved genetic regions bacteria need to survive, creating double-stranded DNA breaks that trigger bacterial death.
The precision is remarkable.
These engineered phages are designed to selectively target resistant E. coli while largely sparing the rest of the gut microbiome - something broad-spectrum antibiotics cannot do.
Natural bacteriophages often have narrow host ranges and face bacterial resistance through multiple mechanisms. The CRISPR-enhanced versions address these limitations through modified tail fiber proteins that broaden bacterial targeting, anti-CRISPR genes that overcome bacterial defenses, and programming that attacks multiple conserved genetic sequences simultaneously.
Laboratory tests show CRISPR-armed phages kill bacteria significantly faster than natural phages. In mouse models, engineered phages reduce bacterial loads more effectively than their wild-type ancestors and trigger less resistance because they attack multiple essential genes at once.
First human data validates the approach
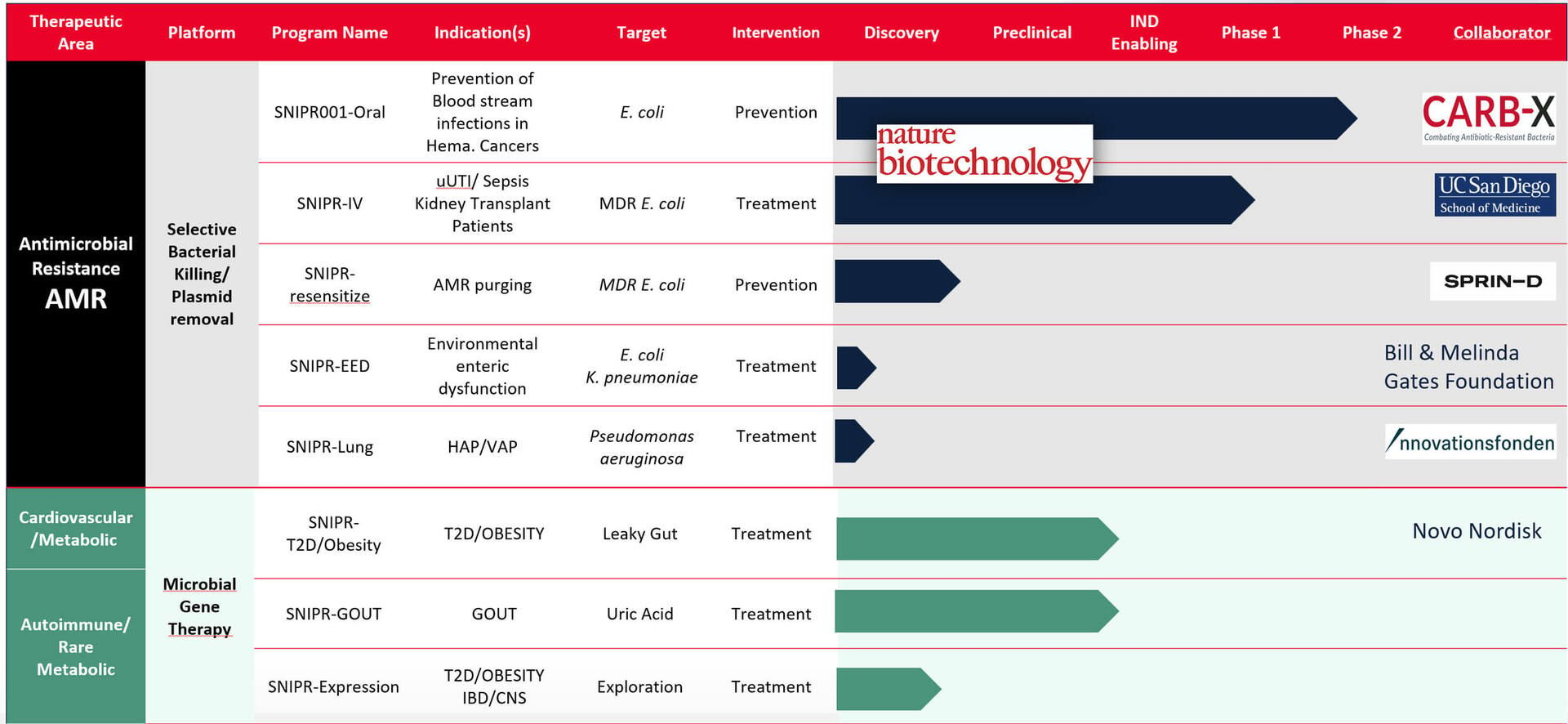
SNIPR Biome, a Danish company, brought the first CRISPR-armed phage to human trials.
Their lead candidate, SNIPR001, targets fluoroquinolone-resistant E. coli in the gut - bacteria responsible for 25–30% of bloodstream infections in cancer patients undergoing chemotherapy.
Researchers screened 162 wild-type phages against 429 diverse E. coli strains, then engineered four phages with modified tail fibers and CRISPR-Cas3 systems targeting resistance genes. The final cocktail covers over 95% of current E. coli clinical isolates.
Phase 1 results published in Nature Biotechnology in 2023 were promising. Among 36 healthy volunteers receiving SNIPR001 over seven days, the treatment was well-tolerated with only mild side effects and no withdrawals. It reduced E. coli levels while sparing the rest of the gut microbiome.
In June 2025, SNIPR began dosing cancer patients in a Phase 1b trial across eight US centers. The study is evaluating SNIPR001 in 24 patients with blood cancers undergoing stem cell transplants - the population most at risk from E. coli bloodstream infections.
Another CRISPR-phage company, Locus Biosciences developed LBP-EC01 for E. coli urinary tract infections.
Their CRISPR-Cas3-enhanced cocktail uses six bacteriophages with both natural bacteria-killing activity and DNA-targeting precision.
Phase 2 ELIMINATE trial results, published in The Lancet Infectious Diseases in August 2024, were promising. Among 39 adult women with uncomplicated UTIs caused by drug-resistant E. coli, the selected dosing regimen showed acceptable tolerability and high drug exposure at the infection site. No serious adverse events occurred, and no genetic resistance emerged in any recovered E. coli samples.
Clinical progress signals market momentum
SNIPR Biome and Locus Biosciences represent the most advanced players in CRISPR-enhanced phage therapy, with both companies progressing candidates through clinical trials and securing significant funding from government agencies and nonprofits focused on combating antibiotic resistance.
BiomX offers a different trajectory. The company is advancing natural phage therapy through clinical development, reporting positive Phase 2 results for BX211 in diabetic foot osteomyelitis in April 2025. BiomX has publicly stated plans to incorporate genome engineering techniques like CRISPR once they establish clinical validation with their current approach.
The field faces real challenges alongside its progress. PHAXIAM Therapeutics entered receivership in 2025, underscoring the financial pressures on phage therapy companies despite growing scientific and clinical interest.
Market analysts estimate the current phage therapy market at $1.1 to $1.3 billion, with projections for substantial growth through 2030. Approximately 90 clinical trials of bacteriophage therapies are underway worldwide, including 41 currently active in the United States, reflecting both scientific momentum and sustained investor confidence in the field's potential to address antibiotic resistance.
What's next: three, five, and ten years out
In three years, expect Phase 3 trial results for the first CRISPR-armed phage products. SNIPR001 and LBP-EC01 will have generated sufficient data to support regulatory submissions. Manufacturing processes will become more standardized.
Within five years, the first CRISPR-enhanced phage therapy could receive FDA approval, likely for high-risk patients where antibiotic options have failed. This will establish regulatory precedent for subsequent products. Diagnostic platforms for rapid bacterial identification and phage susceptibility testing will become commercially available.
A decade from now, CRISPR-enhanced phage therapy will likely be an established treatment option for specific multidrug-resistant infections. Phage cocktails will be stocked in hospital pharmacies alongside antibiotics. Combination therapy protocols using phages and antibiotics together will be standard. AI platforms will predict optimal phage-pathogen matches for personalized treatment.
Prophylactic applications represent another transformative possibility. Patients at high risk for surgical site infections could receive phage applications targeting dangerous resistant bacteria before procedures.
Why this time is different
Phage therapy has been promised for decades. Several factors distinguish this era from past attempts.
The antibiotic crisis is undeniable. Physicians encounter untreatable infections routinely, creating desperate demand for alternatives. Synthetic biology and genetic engineering tools have matured enormously. Regulatory agencies are actively creating pathways for live biotherapeutics. Major funding sources are specifically supporting phage development with substantial capital.
Most importantly, we now have rigorous human clinical data. The Phase 1 and Phase 2 results from SNIPR001 and LBP-EC01 represent genuine milestones - controlled trials with measurable outcomes, not anecdotes.
CRISPR-enhanced phage therapy represents one of our best strategies for preserving antibiotic effectiveness by targeting the resistant bacteria that threaten them. For millions facing infections that no longer respond to traditional antibiotics, these engineered bacteriophages might be exactly the precision strike needed.
Innovation highlights
🦠 AI detects outbreaks first. Asia's PathGen platform spots disease threats before they explode by fusing genomic data with climate patterns, mosquito habitats, and patient information across borders. Countries keep full control of their data while sharing critical insights that could save lives. The AI-powered system promises faster, smarter decisions on treatments and vaccines when minutes matter most. Early pilots launch in 2026 across six Asian countries. Backed by the Gates Foundation and Temasek Foundation, PathGen could slash response times and guide resources exactly where they're needed.
🧠 Injectable electrodes zap tumors. Researchers developed injectable nanoparticles that form electrically conductive hydrogels inside glioblastoma tumors, eliminating the need for risky brain surgery to place metal electrodes. The particles are injected via thin syringe directly into tumor cavities after operation, then deliver irreversible electroporation pulses that destroy cancer cells and activate the immune system. In animal models, tumors were wiped out within three days. The biodegradable electrodes disappear naturally after 12 weeks with no side effects, offering new hope for treating this aggressive brain cancer.
🤖 AI cooks up new antibiotics. MIT researchers trained AI to design completely new antibiotic molecules from scratch—generating 29 million novel compounds in just days. Traditional drug discovery takes years screening existing libraries, but this generative AI approach creates entirely new structures that don't exist anywhere. The team synthesized 22 samples, and one successfully cleared drug-resistant MRSA in mice. With antibiotic resistance killing over a million people annually, this breakthrough could finally address the decades-long discovery gap in new antibiotics.
Cool tool
🌐 ChatGPT Atlas is OpenAI's desktop browser with ChatGPT built into a sidebar. Ask questions about the page you're on, summarize articles, or compare products without copy-pasting between tabs and a chatbot window.
The "agent mode" preview lets ChatGPT perform browser actions like opening tabs or clicking buttons while you supervise. "Browser Memories" can remember sites or facts from your browsing to provide better context later - useful if you're researching products or tracking information across sessions.
I found it especially helpful to search for specific posts on LinkedIn, and for getting advice on how to improve my website designs.
Right now it's macOS only, with Windows and mobile versions expected soon. It's an option if you want AI assistance integrated into your browser rather than as a separate tool, particularly for research, comparison shopping, or automating repetitive online tasks.
Weird and wonderful
Couture meets psoriasis protection. Fashion editor Tallulah Harlech spent years literally counting down minutes at glitzy events until she could sprint home, rip off her designer clothes, and dive into Epsom salt baths. Her guttate psoriasis - "raindrops from hell" - felt like mini burns spreading across her skin in real-time.
After decades of hiding in baggy cotton tees like a "14-year-old skater boy," she created Sylva: high-fashion armor made from seaweed and wood pulp.
The Pyratex seacell 15 fabric feels almost moist to touch, soothes angry skin with antioxidants, and actually survives the washing machine (because psoriasis life means constant cream coverage). The sleek bodycon pieces sport laser-finished seams, extra-long sleeves, and stirrup leggings fancy enough that Daphne Guinness pairs them with heel-less boots. Tallulah’s mum even wore them under vintage Chanel to the Met Gala!
Thank you for reading the Healthy Innovations newsletter!
Keep an eye out for next week’s issue, where I will highlight the healthcare innovations you need to know about.
Have a great week!
Alison ✨
P.S. If you enjoyed reading the Healthy Innovations newsletter, please subscribe so I know the content is valuable to you!