Welcome back to Healthy Innovations! 👋
In this issue, we're exploring how scientists are tackling antimicrobial resistance (AMR), one of healthcare's most pressing challenges.
Picture this: A patient arrives at the emergency room with a severe antibiotic-resistant infection. Instead of limited options, doctors quickly identify the exact bacteria and administer a treatment precisely designed for that specific strain.
Within days, the infection disappears.
This isn't science fiction - it's becoming reality through groundbreaking approaches that are revolutionizing our fight against antibiotic resistance. While major challenges remain, researchers are making remarkable progress with solutions that go far beyond traditional antibiotic development.
So, let’s dive in!
The resistance reality
It is estimated that bacterial antimicrobial resistance was directly responsible for 1.27 million global deaths in 2019 and contributed to 4.95 million deaths.
Recent data from England reveals both encouraging progress and troubling setbacks with regards to AMR.
A comprehensive study of ceftazidime/avibactam (Avycaz by AbbVie) - one of the UK's newest antibiotics launched in 2017 - found that while overall resistance rates remain low at 6.3%, a worrying 89.3% of resistant cases stemmed from specific genetic mechanisms that render the bacteria virtually untreatable.
Even more concerning, researchers documented cases where resistance developed during treatment itself, demonstrating bacteria's remarkable ability to adapt in real-time.
The global nature of this threat became starkly apparent with recent data showing concerning increases in drug-resistant gonorrhea across England. In just the first five months of 2025, 14 cases of ceftriaxone-resistant strains were identified - already surpassing the total for the previous year. Six of these cases showed extensive drug resistance, meaning they failed to respond to multiple treatment options.
Most troubling? These drug-resistant cases are linked with travel to or from the Asia-Pacific region, illustrating how resistance can spread rapidly across continents through international travel. This global connectivity means that resistance emerging in one region can quickly become a worldwide problem.
The innovation revolution
Despite these challenges, there are exciting breakthroughs in the fight against antimicrobial resistance.
We're entering a transformative chapter, where scientists are pioneering innovative approaches that move beyond conventional methods.
Bacteriophage therapy: Nature's smart weapons
Think of this as using "good" viruses to fight bad bacteria.
These natural defenders can be customized to target harmful bacteria while leaving beneficial bacteria untouched - like having a smart missile instead of a bomb.
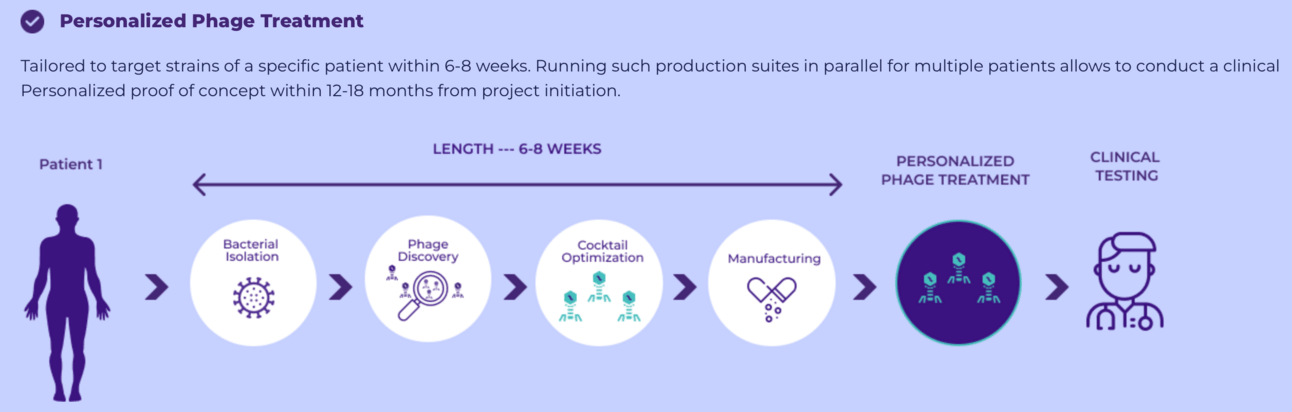
BiomX is a clinical-stage biotechnology company specializing in the development of bacteriophage (phage) therapies - both natural and engineered - as well as personalized phage treatments. These therapies are designed to target and destroy harmful bacteria implicated in chronic diseases with significant unmet medical needs, such as cystic fibrosis (CF) and diabetic foot osteomyelitis (DFO)
AI-powered drug discovery
Instead of scientists spending years testing different compounds in the lab, computers can now quickly scan through millions of possibilities to find promising new treatments.
The breakthrough example?
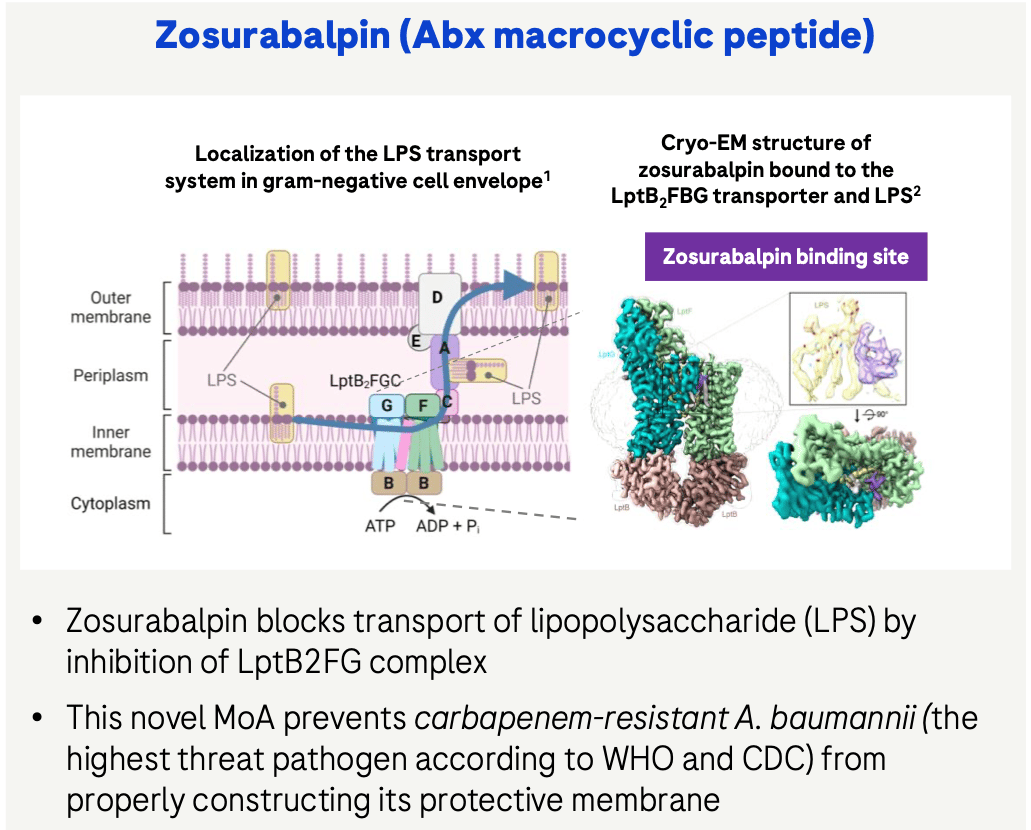
Zosurabalpin - the first new antibiotic in 50 years that can fight a particularly dangerous superbug. Instead of attacking bacteria directly, it cleverly disables the bacteria's protective shield - a completely new way of thinking about treatment.
Roche has taken the bold step of advancing zosurabalpin through final-stage trials despite the challenging economics of antibiotic development. As Michael Lobritz, Roche's global head of infectious diseases, emphasizes: "Our goal is to contribute new innovations to overcome antimicrobial resistance, one of the biggest infectious disease challenges to public health."
Rapid diagnostics: Speed saves lives
Perhaps most exciting is how quickly we can now identify which bacteria are causing an infection.
New testing methods can give doctors this information within hours rather than days, helping them choose the right treatment from the start and preventing the desperate cycle of trial-and-error treatment that often drives resistance.
The access challenge
However, breakthrough science alone won't solve the crisis.
A recent study, published in Lancet Infectious Diseases, examined eight countries: Bangladesh, Brazil, Egypt, India, Kenya, Mexico, Pakistan, and South Africa. The findings revealed that only 6.9% of patients with carbapenem-resistant infections received appropriate treatment. Even in India, which obtained 80% of the available antibiotics in the study, just 7.8% of estimated cases received proper treatment.
Dr. Abdul Ghafur, an infectious disease consultant at Apollo Hospital in Chennai, puts it bluntly: "We often see patients for whom no antibiotic works - and they die." He describes a cruel paradox: wealthy patients overuse antibiotics while poor patients can't access them at all.
Dr. Ghafur advocates for systematic changes: "Ideally, every antibiotic prescription in hospitals should require a second sign-off - by an infection specialist or microbiologist. Some hospitals do this, but most don't." Such oversight could prevent both resistance development and treatment failures.
A 2024 Lancet publication projects that by 2050, antimicrobial resistance (AMR) will directly cause 1.91 million annual deaths globally and contribute to 8.22 million deaths. The authors conclude that "it is important that interventions combine infection prevention, vaccination, minimization of inappropriate antibiotic use in farming and humans, and research into new antibiotics to mitigate the number of AMR deaths that are forecasted for 2050.”

Prediction: Death rate attributable to AMR, all ages, 2050
What the next decade holds
The next 3 years: Bacteriophage therapy will expand from research settings to routine clinical applications. Hospitals will develop phage treatment protocols for specific types of resistant infections, particularly those involving chronic wounds and medical device-associated biofilms. AI-designed antimicrobials will complete their first major clinical trials, with zosurabalpin leading the way.
Five years from now: Personalized antimicrobial treatment will become standard practice. Custom bacteriophage preparations will be developed for individual patients, with specialized facilities producing tailored viral treatments for specific infection profiles. Advanced diagnostic systems will not only identify resistant bacteria but also predict which patients are most likely to develop resistance during treatment.
A decade into the future: The treatment of bacterial infections will look radically different. AI systems will continuously monitor bacterial evolution patterns globally, helping predict resistance development and guide the creation of new treatments before resistant strains become widespread. Engineered therapeutic bacteria may provide real-time monitoring and treatment of infections from within the body.
Looking ahead with measured optimism
The advances in combating antibiotic resistance represent more than just new drug development - they reflect a fundamental shift in how we approach infectious disease treatment.
From BiomX's engineered bacteriophage platforms that create programmable phages with improved targeting accuracy, to AI systems that can predict resistance patterns before they emerge, we're witnessing a convergence of technologies that gives us genuine reason for optimism.
The progress being made shows we can stay ahead of bacterial resistance, but only if we can bridge the gap between innovation and access. The race against superbugs isn't just about creating better weapons - it's about ensuring those weapons reach everyone who needs them, anywhere in the world.
Enjoying Healthy Innovations? Forward this to a colleague who needs to stay two steps ahead.
📬 Not subscribed yet? Sign up here to receive future editions directly.
Innovation highlights
🩻 Meet CLAIRITY BREAST, the FDA's newly approved prognostic AI tool that analyzes routine mammograms to predict breast cancer risk over the next five years by spotting patterns that even experienced radiologists might miss. Trained on millions of images and tested across 77,000 diverse mammograms, this tool could transform standard screenings into personalized risk assessments that could revolutionize early detection and prevention strategies.
🔬 PanDerm represents a breakthrough in dermatological AI by simultaneously analyzing multiple image types - close-up photos, dermoscopy images, pathology slides, and total body photographs. Trained on over 2 million skin images from 11 international institutions, this sophisticated tool improved melanoma diagnostic accuracy by 11% and boosted detection of other skin conditions by 16.5%. With skin conditions affecting 70% of the global population, PanDerm's ability to synthesize diverse visual data could significantly enhance early detection capabilities, particularly in resource-limited settings where dermatologist access is restricted.
Company to watch
Owkin is a pioneering AI biotech company founded in 2016 that uses AI to speed up drug discovery, reduce clinical trial risks, and develop better diagnostic tools to improve patient outcomes. The company works with leading academic centers and pharmaceutical companies (Sanofi, BMS and MSD), using advanced machine learning on complex patient data to find new drug candidates, identify biomarkers, and deliver precision medicine.
At the heart of Owkin's approach is privacy-preserving federated learning—a system that lets AI models learn from data across multiple institutions while keeping patient information secure. They also focus on building rich datasets that capture the full complexity of disease biology. Their technology not only helps identify new treatments but also creates AI diagnostic tools that integrate smoothly into digital pathology workflows, helping doctors screen biomarkers and predict treatment outcomes in cancer care.

Owkin
Weird and wonderful
🚀 Meet Osteoboost, the world's first FDA-approved vibrating belt that looks like a '90s tourist accessory but packs NASA-level bone-saving technology! This isn't your infomercial abs-zapping gadget - this unassuming waist pouch delivers precisely 30 hertz of gentle oscillations to combat osteopenia (low bone density) in postmenopausal women. Born from NASA's discovery that zero gravity literally sucks the calcium out of astronauts' bones, this $995 device mimics weight-bearing exercise while you make coffee. Clinical trials showed an impressive 85% reduction in bone loss, proving that sometimes the most effective medical breakthroughs come disguised as tourist gear.
Thank you for reading the Healthy Innovations newsletter!
Keep an eye out for next week’s issue, where I will highlight the healthcare innovations you need to know about.
Have a great week!
Alison ✨
P.S. If you enjoyed reading the Healthy Innovations newsletter, please subscribe so I know the content is valuable to you!
P.P.S. Healthcare is evolving at an unprecedented pace, and your unique insights could be invaluable to others in the field. If you're considering starting your own newsletter to share your expertise and build a community around your healthcare niche, check out beehiiv (affiliate link). There's never been a better time to start sharing your knowledge with the world!