Welcome back to Healthy Innovations! 👋
In this issue of Healthy Innovations we're exploring synthetic health data - a clever solution to healthcare's data dilemma.
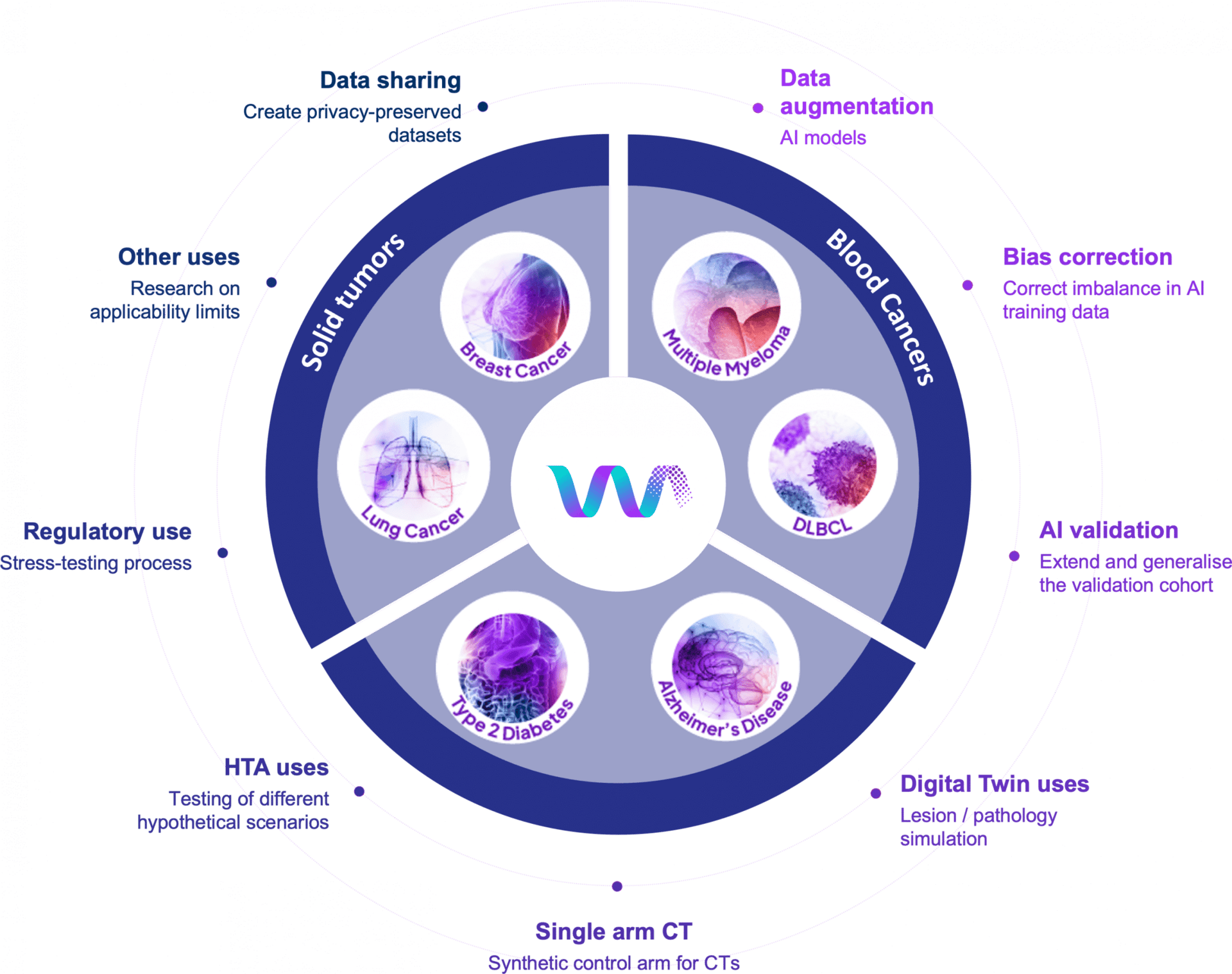
For years, researchers needed patient information to advance medicine, but privacy laws (rightly) kept that data locked away. Synthetic health data changes the game by creating artificial patient records that look and act like real clinical data, without containing any actual patient information.
Let's dive in!
Healthcare is solving one of its toughest challenges. We need massive amounts of patient information to train AI systems that could revolutionize diagnosis, drug development, and clinical care. Privacy laws like HIPAA and GDPR protect that data behind secure walls, exactly as they should - patient confidentiality matters.
Synthetic health data offers an elegant solution: artificially generated patient records that capture real clinical patterns without containing information from actual people. These "realistic but not real" datasets allow researchers to experiment more freely, AI systems to train with reduced privacy constraints, and hospitals to collaborate in ways previously difficult to achieve.
Breaking down data barriers
Healthcare AI needs enormous datasets to function properly, but high-quality clinical data has historically been difficult to access. Privacy regulations, along with contractual, technical, and governance barriers, strictly control data sharing. Medical datasets often skew toward acute care settings like ICUs, leaving chronic illness management, outpatient care, and diverse populations underrepresented.
Synthetic data can significantly change this equation. By creating artificial patient records that preserve statistical relationships without revealing individual identities, researchers can access broader data while maintaining strong privacy protections.
Real innovation happening now
Companies like MDClone, Syntegra, Synthia and Syntho have built platforms that generate synthetic datasets from real patient populations, statistically matching original data while removing identifying information.
MDClone has raised over $100 million and partners with the National Institutes of Health and Department of Veterans Affairs. Their platform can reduce data access times from months to days, according to customer testimonials.
Syntegra generated synthetic versions of 2.6 billion rows of data from over 413,000 COVID-19 patients for the NIH's National COVID Cohort Collaborative, expanding access to pandemic research while protecting patient privacy. The Bill and Melinda Gates Foundation has contracted with Syntegra to create synthetic versions of clinical trial data for HIV and maternal health programs.
The applications are expanding across healthcare.
Pharmaceutical companies can accelerate drug development timelines by accessing diverse patient populations for real-world evidence studies. Clinical trial design improves with synthetic cohorts that help identify optimal endpoints before investing in actual trials.
For clinicians, synthetic data enables AI diagnostic tools trained on broader, more representative datasets. These tools can learn from rare conditions and diverse patient populations that individual physicians might never encounter, leading to more accurate predictions for complex cases.
For patients, synthetic data allows their medical experiences to contribute to progress while greatly reducing re-identification risk. This enables research into rare diseases that might otherwise lack sufficient patient numbers for meaningful study.
Additional benefits include:
Institutional collaboration – Health systems can share insights across organizations with reduced governance overhead
Accelerated research – Scientists can conduct exploratory analysis and validate algorithms more rapidly
Digital health innovation – Companies can build and test new technologies with reduced privacy concerns
Learning to use it wisely
Like any powerful technology, synthetic data works best when used thoughtfully. Studies show that when AI systems train exclusively on synthetic data through many iterations, quality can degrade - a phenomenon researchers call "model collapse." This discovery has guided the field toward smarter approaches.
Rather than viewing synthetic data as a complete replacement for real patient information, leading researchers use it strategically to fill specific gaps - rare genetic syndromes, underrepresented demographics, edge cases - while real patient data provides the anchor.
Building the right framework
The synthetic data field is maturing, with companies and institutions developing governance structures that maximize benefits while managing risks.
Leading vendors emphasize validation and privacy assessment. Syntegra's documentation references third-party privacy evaluation and statistical fidelity testing. MDClone's platform undergoes continuous validation by health systems using it, creating a feedback loop that improves quality.
Healthcare organizations are increasingly adopting best practices:
Tag datasets with metadata showing source and validation history
Set reasonable ratios between synthetic and real data in training sets
Use automated tools to monitor alignment with real-world patterns
The approach shows promise. Institutions report that combining synthetic data for rapid exploration with real data for validation offers speed and accessibility alongside reliability.
Regulatory frameworks are evolving. The FDA has expressed interest in how synthetic data could inform approval decisions, and guidance is emerging that acknowledges its role while ensuring appropriate oversight.
The road ahead
Synthetic health data is solving a problem that's frustrated researchers for years: how to access the information they need while keeping patient privacy sacred.
The impact is already tangible. Rare disease researchers who once struggled to find enough patients can now create synthetic cohorts to test their hypotheses. Health systems across different countries are collaborating without the usual bureaucratic tangles. Startups are building healthcare AI without spending months navigating data access approvals.
We're at an inflection point where synthetic data has moved from "interesting experiment" to "practical tool" that major institutions rely on daily. Here's what makes this work: synthetic data isn't trying to replace real patient information. It's filling in the gaps and speeding up exploration, while real-world data keeps everything grounded and accurate.
Healthcare has always involved trade-offs between innovation and protection. Synthetic health data is showing us we don't always have to choose. Sometimes, with smart technology and thoughtful implementation, we get to have both.
Innovation highlights
🥲 Chronic pain's energy crisis. Duke researchers discovered that support cells called satellite glia ship fresh mitochondria to nerve cells through tiny tubes, keeping pain-sensing nerves functioning properly. When this delivery system breaks down - from diabetes or chemotherapy - nerves run out of power and start firing randomly, triggering chronic pain. In mice, transferring healthy glia relieved nerve damage pain by restoring the mitochondrial supply. The finding opens possibilities for new treatments: either boosting glia's natural delivery or directly injecting lab-grown mitochondria into damaged nerves.
🧬 Gene editing goes scissors-free. Scientists at UNSW in Sydney developed a new CRISPR technique that switches genes back on without cutting DNA. Instead of snipping genetic material, it removes chemical "brakes" called methyl groups that keep certain genes silent. The method aims to offer a safer path to treating Sickle Cell disease by reawakening fetal hemoglobin production. Lab tests in human cells showed promising results, with researchers now planning further preclinical development toward potential clinical trials.
🧠 Your tumor's virtual stunt double. University of Michigan researchers built a "digital twin" for brain tumors that models patient-specific metabolism using machine learning trained on clinical and experimental data. The virtual replica can simulate how a tumor's metabolism responds to candidate metabolic therapies, with predictions validated against human samples and mouse experiments. This research-stage platform points toward a future where doctors could trial strategies on a patient's digital copy first, potentially sparing them ineffective treatments.
💊 Cancer care gets a custom fit. A UC San Diego clinical trial called I-PREDICT found that tailoring cancer drug combinations to each patient's specific tumor mutations was associated with significantly better outcomes than less-matched regimens. Using genomic sequencing and a molecular tumor board, doctors created personalized multi-drug plans for each person. By starting novel combinations at reduced doses and escalating carefully, the team showed these regimens could be delivered with acceptable safety, supporting what researchers call a shift toward "one-size-fits-one" oncology.
Company to watch
🧬 OutSee is a Cambridge, UK based genomics company using AI to discover drug targets in ways traditional methods miss.
Their Nomaly platform takes a "genomics first" approach, predicting disease directly from a single genome rather than relying on conventional association-based analysis. This lets researchers extract fresh insights from already-analyzed datasets and smaller cohorts that standard statistical tools struggle with.
Founded in 2023 by Dr. Julian Gough (formerly of the MRC Laboratory of Molecular Biology), the company recently closed a £2.5M seed round led by Ahren Innovation Capital. In January 2026, OutSee announced a strategic partnership with o2h discovery to advance their lead target into active drug development.
The platform already works with major genomics initiatives including Genomics England and FinnGen to uncover disease-modifying mechanisms from population-scale data. For drug discovery teams facing diminishing returns from traditional genomic analysis, OutSee offers a compelling new lens on familiar datasets.
Weird and wonderful
💤 Sleep like an Egyptian pharaoh. Finally, a sleep gadget that looks like it belongs in a museum gift shop but actually does something useful! Serapis combines white noise, breathing lights, fractal visuals, and something called Schumann Resonance (7.83Hz brain-wave syncing, naturally) into one pyramid-shaped bedside companion.
The device runs a 2-minute quiz to figure out your sleep saboteur - overthinking, jet lag, noise sensitivity - then customizes its light-and-sound cocktail accordingly. Press one button, choose 30 or 60 minutes, and let the $144 pyramid work its magic without ever touching your phone. At 1.2kg of metal and plastic pharaoh energy, it's hefty enough to feel legitimate and weird enough to spark bedroom conversation.
Ancient geometry meets modern insomnia!
Thank you for reading the Healthy Innovations newsletter!
Keep an eye out for next week’s issue, where I will highlight the healthcare innovations you need to know about.
Have a great week!
Alison ✨
P.S. If you enjoyed reading the Healthy Innovations newsletter, please subscribe so I know the content is valuable to you!
P.P.S. If your New Year’s resolution was to start your own newsletter, check out beehiiv (affiliate link). There's never been a better time to start sharing your knowledge with the world!