Welcome back to Healthy Innovations! 👋
What if your next life-saving medical device wasn't hardware at all, but software running on a phone? In this week's deep dive, we unpack Software as a Medical Device (SaMD) – AI-powered software that's earning FDA clearance, cutting stroke treatment times, predicting sepsis before symptoms appear, and reshaping what "medical device" even means.
Let’s dive in!
Your next prescription might not come in a bottle. It might be a download.
Software as a Medical Device (SaMD) is one of the fastest-growing categories in healthcare. These aren't wellness apps that count your steps. They're clinically validated, FDA-regulated software programs that function as standalone medical devices, running on smartphones, laptops, and cloud servers rather than specialized medical hardware.
In 2025, the FDA cleared roughly 300 AI-enabled medical devices, and 62% of them were SaMD. Over 1,250 AI-enabled devices are now authorized for marketing in the United States. The shift from hardware to software in healthcare is well past the proof-of-concept stage.
SaMD vs. SiMD: A critical distinction
The International Medical Device Regulators Forum (IMDRF) defines SaMD as "software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device."
SaMD operates independently – an AI algorithm that analyzes CT scans for stroke, or a mobile app that adjusts insulin recommendations. The software itself is the device. This is different from Software in a Medical Device (SiMD), which is embedded software that powers hardware, like the firmware inside a pacemaker.
Because SaMD can be deployed globally with a software update – no factory line, no shipping container – the market is projected to grow several-fold between the mid-2020s and early 2030s, fueled by AI, cloud computing, and the expansion of remote care.
Where SaMD is saving lives right now
The most mature applications are in medical imaging, which accounts for over 71% of all AI/ML device clearances. But SaMD is expanding rapidly across cardiology, oncology, mental health, and chronic disease management.
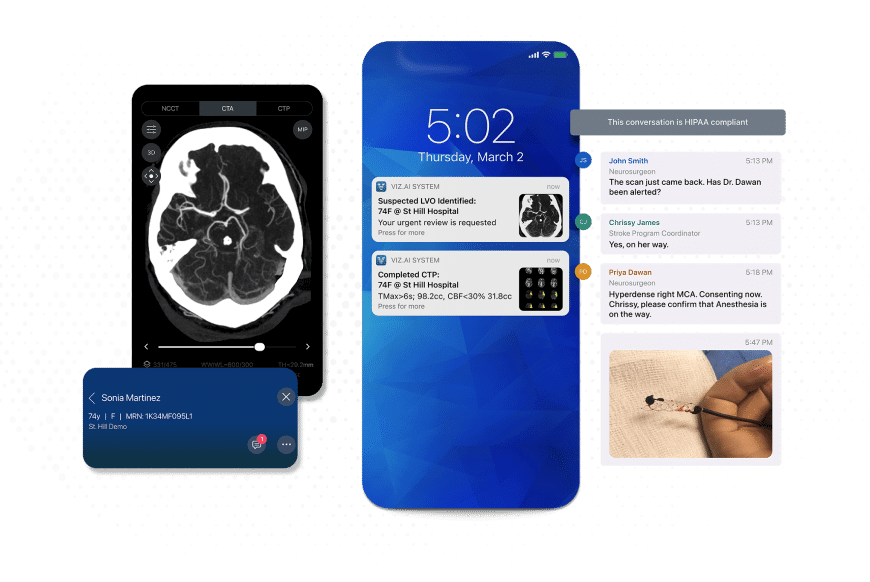
Stroke detection: Viz.ai's AI-powered platform is deployed across more than 1,600 hospitals, with multiple FDA-cleared algorithms spanning neurovascular, cardiovascular, vascular, and trauma applications. In a multicenter study presented at the 2025 International Stroke Conference, it reduced stroke treatment time by an average of 31 minutes. In stroke care, each minute of delay in endovascular therapy can translate into several days of disability-adjusted life lost. Other clinical data show a 40% reduction in disability at 90 days.
Sepsis prediction: Prenosis received FDA De Novo marketing authorization in 2024 for Sepsis ImmunoScore, an AI tool that analyzes 22 diverse parameters, combining biomarkers and clinical data, to assess a patient's risk of sepsis within 24 hours. It integrates directly with electronic health records.
Diabetic retinopathy screening: IDx-DR (now LumineticsCore) made history in 2018 as the first fully autonomous AI diagnostic cleared by the FDA, screening for diabetic retinopathy without a specialist present, with sensitivity and specificity in the high-80s to low-90s. It brought specialist-level eye screening into primary care offices for the first time.
Digital therapeutics: Prescription software-based treatments are supplementing pharmaceuticals. Akili Interactive's EndeavorRx was the first FDA-cleared video game therapy for pediatric ADHD in 2020, while programs targeting substance use disorder and diabetes management continue expanding the category.
The AI acceleration
Foundation models are the next frontier in SaMD.
Aidoc, whose enterprise-level aiOS platform holds 18 FDA clearances covering conditions from stroke to pulmonary embolism and cervical fractures, received funding in 2025 to develop its CARE (Clinical AI Research Engine) foundation model. Rather than training separate algorithms for each condition, CARE learns broad patterns from massive datasets and applies them across multiple clinical applications.
Meanwhile, Tempus acquired digital pathology AI company Paige in August 2025, with CEO Eric Lefkofsky saying the deal "substantially accelerates" their effort to build the largest foundation model in oncology. Tempus combines one of the world's largest clinical and molecular datasets with AI analytics for precision medicine.
The regulatory game changer
Traditional regulations required a new submission for every significant software modification, creating bottlenecks that could delay critical updates by months. Predetermined Change Control Plans (PCCPs) are changing that.
The PCCP concept has international backing. In October 2023, the FDA, Health Canada, and the UK's MHRA jointly published five guiding principles for PCCPs in machine learning-enabled medical devices, establishing that such plans should be focused and bounded, risk-based, evidence-based, transparent, and managed across a device's total product lifecycle. By early 2025, the FDA finalized its own detailed PCCP guidance for AI-enabled device software functions.
A PCCP allows manufacturers to map out anticipated software modifications at the time of their initial regulatory submission. If the plan is authorized, the company can implement those pre-specified updates, including AI model retraining and performance improvements, without filing a new marketing application for each change. Each plan must include three core elements: a description of planned modifications, a modification protocol outlining validation steps, and an impact assessment analyzing how changes affect safety.
This tri-regulator alignment signals that the US, UK, and Canada are moving toward a shared understanding of how AI-based medical software should evolve post-approval, rather than each jurisdiction developing conflicting frameworks.
Global companies to watch
Lunit (Seoul, South Korea): A publicly listed AI cancer diagnostics company serving over 10K healthcare providers across more than 65 countries. Its INSIGHT suite detects chest abnormalities with 97-99% accuracy and breast cancer with 96% accuracy. In 2024, Lunit acquired New Zealand-based Volpara Health Technologies to create a comprehensive cancer intelligence portfolio, and its AI was selected for Australia's national breast cancer screening program, widely described as a global first.
Huma Therapeutics (London, UK): Received FDA Class II clearance in 2023 for a disease-agnostic SaMD platform, meaning it can be applied across multiple conditions rather than being limited to one. The platform integrates with external devices like heart rate monitors and glucose meters, and hosts AI algorithms supporting screening, diagnosis, and clinical decision-making.
XUND (Vienna, Austria): An MDR-certified SaMD platform for digital triage and diagnosis, serving more than 10M patients. XUND raised funding in early 2025 to expand across the DACH region and the UK.
The hurdles ahead
Regulatory fragmentation remains a real barrier. Multi-region SaMD launches can cost several hundred thousand to multiple millions of dollars. The EU's MDR often classifies software at higher risk levels than US guidelines, and global harmonization is still a work in progress.
Algorithmic bias is an ongoing concern. To date, the FDA has only approved AI/ML tools using "locked" algorithms that don't change after deployment. The PCCP framework is beginning to open the door for adaptive models, but training data diversity remains a critical challenge.
Reimbursement uncertainty persists. Even with FDA clearance, insurance coverage for SaMD is inconsistent. Germany's DiGA pathway, which created direct reimbursement for approved digital therapeutics, is often cited as a model other markets could follow.
FDA capacity constraints add another layer of risk, with double-digit workforce reductions in 2025 even as AI-enabled submissions surge.
Despite these challenges, the results speak for themselves.
Software algorithms are already detecting strokes faster than radiologists, predicting sepsis hours before symptoms surface, and screening for eye disease in clinics that have never had a specialist on site. Patients who would have waited weeks for a diagnosis are getting answers in minutes. Communities that lacked access to specialist care are getting it through a screen.
The definition of "medical device" has fundamentally expanded. Your next life-saving device might already be in your pocket. It just needs the right algorithm.
Innovation highlights
🤖 AI beats BMI. Researchers at the University of Tokyo built an AI tool called AI-IR that predicts insulin resistance using just nine pieces of standard health checkup data. Applied to 500K UK Biobank participants, it outperformed BMI at identifying people at risk for 12 different cancers linked to insulin resistance. The tool catches cases BMI misses — metabolically unhealthy people at a "normal" weight — and could slot right into routine screenings for diabetes, heart disease and cancer.
🥇 Gold nanoparticles read blood. Researchers at St. Petersburg University built a laser-based method that detects neopterin – a key immunity biomarker – directly in blood serum within minutes. Neopterin levels spike during viral infections, autoimmune diseases, and organ transplant rejection, but traditional detection methods require complex prep and significant time. The new approach uses gold nanoparticles that amplify the molecule's optical signal millions of times, clearly picking it out among countless other blood compounds. Results come back almost immediately.
🔮 Catching cancer before it catches you. Carnegie Mellon spinout Xlue Inc. is training AI on millions of patient medical records to spot who's at high risk for lung, liver, and pancreatic cancer – before symptoms even show up. Their CATCH-FM tool predicts cancer risk by tracking patterns in a patient's medical history over time. For patients with no prior cancer history, it hit 50% accuracy; for those with previous diagnoses, that jumped to 70%.
Cool tool
🍌 Nano Banana Pro is Google's latest AI image generator, and it's a genuine step up for anyone who creates visual content. Built on the Gemini architecture, it produces studio-quality images with something most AI tools still struggle with: text that's actually readable and correctly spelled, even in multiple languages.
You can generate everything from posters and infographics to product mockups in up to 4K resolution, blend up to 14 input images while keeping faces and objects consistent, and make precise edits like swapping objects or tweaking lighting without starting from scratch. A nice bonus: it connects to Google Search, so your diagrams and infographics can reflect real-world data. You can try it through the Gemini app for free (with limits), or go bigger through Google AI Studio and Vertex AI on paid plans.
Weird and wonderful
👃 How good does that photo smell! An MIT researcher named Cyrus Clarke has built a contraption that looks like it belongs on a 1970s sci-fi set – and it turns old photographs into custom fragrances. The Anemoia Device (named after the feeling of nostalgia for a time you never lived) uses a built-in AI to analyze a photo and generate a caption, then lets you twist three physical dials to adjust the subject, age, and mood. Clarke describes the dials as "more akin to tuning an instrument" than wrestling with a blank AI prompt.
A language model converts all of that into a short poem, then picks from a library of 50 scents to mix your memory in a glass beaker. Feed it a photo of the Great Wall of China and you'll get campfire, dirt, cedar, and bamboo. Feed it a beach snapshot from the '80s and you'll presumably get sunscreen and questionable fashion choices. Finally, a gadget that makes your vacation slides interesting – and sniffable.
Thank you for reading the Healthy Innovations newsletter!
Keep an eye out for next week’s issue, where I will highlight the healthcare innovations you need to know about.
Have a great week!
Alison ✨
P.S. If you enjoyed reading the Healthy Innovations newsletter, please subscribe so I know the content is valuable to you!