Welcome back to Healthy Innovations! 👋
Your smartwatch says you had three hours of deep sleep last night. Should you believe it?
A recent Associated Press article explored the limits of consumer sleep trackers – and this comes at an interesting moment for sleep medicine. While experts caution against obsessing over nightly scores (there's even a term for that: "orthosomnia"), the broader field is experiencing a genuine transformation.
This week's Deep Dive looks at what's actually working: from the first FDA-approved medication for sleep apnea to AI that can detect disorders from your phone.
Let’s dive in!
GLP-1s for sleep disorders
Sleep apnea affects an estimated one billion people worldwide. For decades, the standard treatment has been a CPAP machine – effective, but notoriously difficult to tolerate. Up to half of patients prescribed CPAP can't stick with it, leaving millions struggling with a condition linked to heart disease, stroke, and cognitive decline.
That changed in late 2024 when the FDA approved Zepbound (tirzepatide) as the first prescription medication specifically indicated for obstructive sleep apnea in adults with obesity.

Image source: Noun Project
The approval followed the SURMOUNT-OSA trials, which enrolled over 400 participants with moderate-to-severe sleep apnea and obesity.
The results were substantial. Patients receiving the dual agonist tirzepatide experienced a reduction in apnea-hypopnea index (AHI) of approximately 25 events per hour. Placebo patients saw a reduction of around 5 events per hour.
"Sleep apnea is now entering an era where medications, not just devices like CPAP, can play a role in treatment," says Dr. Atul Malhotra, a sleep researcher at UC San Diego Health and co-author of the trial.
Beyond reducing breathing interruptions, tirzepatide also lowered high-sensitivity C-reactive protein, a marker of systemic inflammation and cardiovascular risk. This suggests GLP-1 receptor agonists may address sleep apnea through multiple mechanisms: reducing fat deposits around the upper airway, decreasing inflammation, and improving overall metabolic health.
For patients who have failed CPAP, this represents a genuine alternative rather than a compromise.
When a nerve stimulator beats a mask
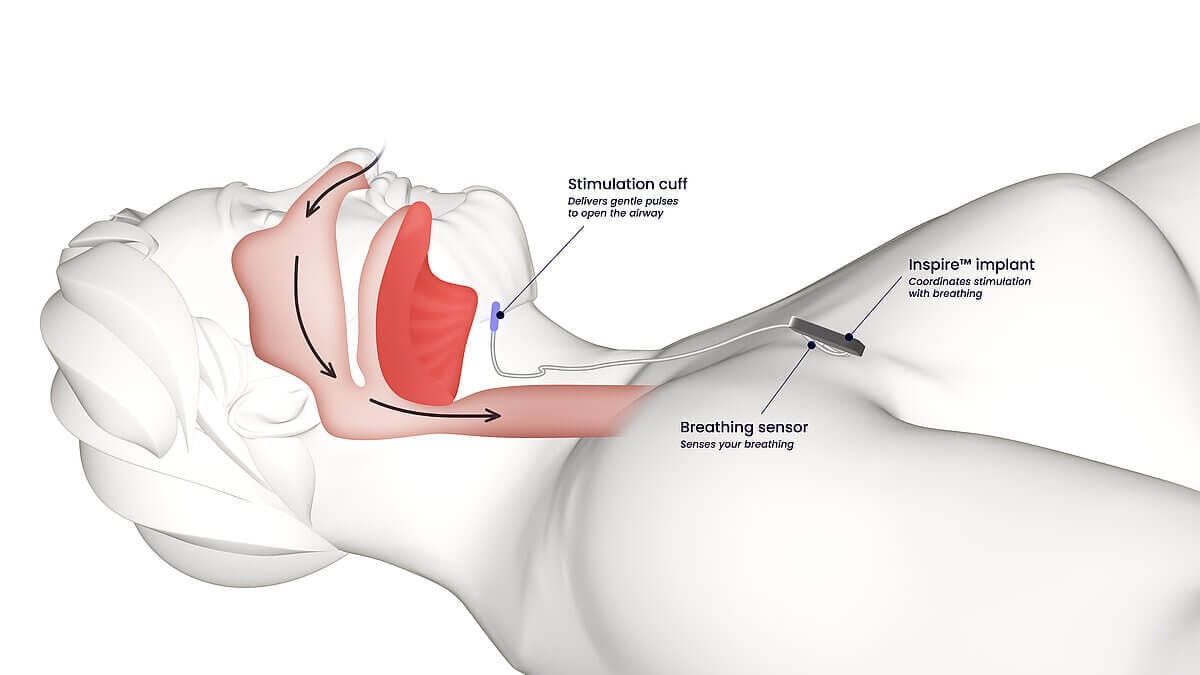
For patients who can't tolerate either CPAP or medications, hypoglossal nerve stimulation offers another path forward.
The Inspire device, approved by the FDA in 2014, works by stimulating the hypoglossal nerve to prevent the tongue from collapsing backward and blocking the airway during sleep. A meta-analysis found Inspire reduced AHI by approximately 20 events per hour in the short term and 16 events per hour over longer follow-up.
Studies report 80-90% of patients experience significant improvement in symptoms. Adherence rates far exceed CPAP – largely because the device requires nothing more than pressing a button on a remote before bed.
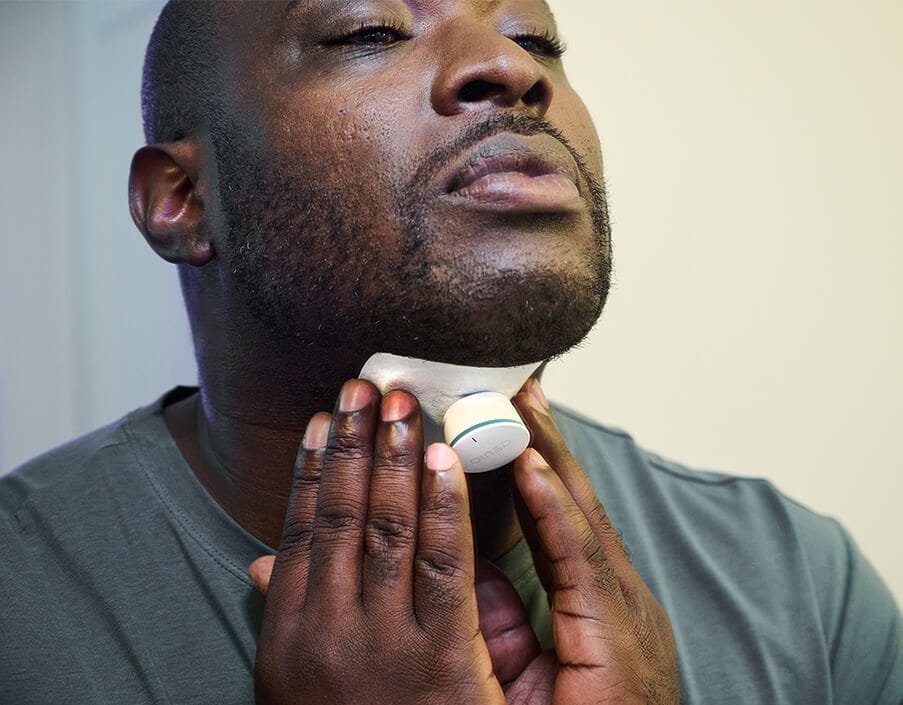
Nyxoah's Genio system received FDA clearance in August 2025, offering a bilateral stimulation approach through a single submental incision rather than the chest-based implant Inspire uses. Early adopters have begun implantation, giving patients and physicians their first real choice in neurostimulation devices for sleep apnea.
The candidacy criteria remain specific: patients must have moderate-to-severe apnea, a BMI under 35-40 depending on the device, and no complete concentric collapse of the soft palate. But for those who qualify, the technology is transforming outcomes.
AI moves from the lab to the bedroom
Artificial intelligence is reshaping sleep medicine in both clinical and consumer settings.
In hospitals and sleep labs, multiple FDA-cleared AI systems now automate sleep study scoring, analyzing polysomnography data with accuracy that rivals trained sleep technologists. EnsoData received the first FDA clearance for AI-based sleep scoring in 2017, with several competitors following since.
Consumer sleep tech: useful, but know the limits
The clinical innovations are paralleled by an explosion in consumer sleep technology.
The U.S. sleep-tracking devices market generated around $5 billion in 2023 and is expected to double by 2030, according to Grand View Research.
Wearable devices dominate the category, accounting for an estimated 72% of the market. The Oura Ring has emerged as a standout, with validation studies showing up to 89% agreement with polysomnography for detecting deep and REM sleep – higher than competing wrist-based devices.
But experts urge users to understand what these devices actually measure.
"The algorithms used by major brands have become highly accurate for determining when someone is asleep," says Daniel Forger, a University of Michigan math professor who researches sleep wearables. "If you really want to know definitively how much non-REM sleep you're having versus REM sleep, that's where the in-lab studies really excel."
Dr. Chantale Branson, a neurologist at the Morehouse School of Medicine, says she frequently sees patients arrive with sleep scores in hand, fixated on granular details such as how much REM sleep they got on a certain night. She advises against this approach: the devices help highlight trends over time but should not be viewed as a definitive measure of sleep health. Nor should any single night's data be seen as significant.
"We would have believed them with or without the device and worked on trying to figure out why they can't sleep – and that is what the wearables do not do," Branson says.
Some users find the data genuinely helpful for behavior change.
Kate Stoye, an Atlanta-area middle school teacher, noticed that the few nights she drank alcohol coincided with poorer sleep quality on her Oura Ring – and decided to give up alcohol as a result. Others become so fixated on their nightly scores that it causes anxiety, a condition researchers have dubbed "orthosomnia."
📊 Orthosomnia (from the Greek "ortho" meaning correct, and "somnia" meaning sleep) describes an unhealthy obsession with achieving perfect sleep scores. The term isn't yet a formal diagnosis, but sleep experts use it to describe people who become so fixated on their tracker data that the anxiety itself disrupts their rest. The telltale sign: feeling distressed about a "bad" sleep score even when you woke up feeling fine.
Smart mattress systems like the Eight Sleep Pod 5 Ultra take a different approach.
The system uses embedded pressure sensors to track sleep stages while actively controlling bed temperature, creating a microclimate between 55 and 110°F. It adjusts throughout the night based on circadian rhythms and sleep stages.
For the subset of poor sleepers whose issues stem from temperature dysregulation – think perimenopausal night sweats or urban heat – thermal control can address the root cause rather than just tracking symptoms.
Prescription apps for insomnia
Digital therapeutics represent another frontier.
Somryst, the first FDA-cleared prescription digital therapeutic for chronic insomnia, delivers cognitive behavioral therapy for insomnia (CBT-I) through a mobile application. Clinical trials showed more than 40% of patients no longer met criteria for insomnia after completing the nine-week program.
SleepioRx, a similar FDA-cleared digital treatment, gained Medicare reimbursement codes in January 2025, opening the door to broader adoption.
These treatments address a critical access problem: CBT-I is the guideline-recommended first-line treatment for chronic insomnia, but qualified therapists are in short supply.
Unlike sleep tracking apps that simply monitor patterns, prescription digital therapeutics actively treat underlying conditions through evidence-based interventions including sleep restriction, stimulus control, and cognitive restructuring.
What's coming next
The pipeline continues to expand.
Investigational oral treatments for sleep apnea – including Apnimed's AD109 and Incannex's IHL-42X, which received FDA Fast Track designation in December 2024 –could further reduce reliance on devices.
Novel PAP technologies like Kairos positive airway pressure (KPAP) show improved comfort and fewer leaks compared to traditional CPAP.
Clinicians now have more treatment options than ever: GLP-1 medications, neurostimulation devices, AI diagnostics, and digital therapeutics. Yet the transformation remains incomplete. Regulatory frameworks continue to evolve, reimbursement stays inconsistent, and questions about long-term effectiveness outside clinical trials persist.
But for the first time, sleep medicine is moving beyond a one-size-fits-all approach toward treatments tailored to individual patients and the interventions most likely to help them.
Innovation highlights
👁️ LASIK for your brain. Scottish researchers have adapted deep-ultraviolet lasers, already used in routine eye surgery, for softer tissues like the brain. The team achieved tissue removal with 10-micrometer precision, far finer than the millimeter-scale accuracy of current neurosurgery. That's 10 times thinner than a human hair, with no detectable damage to surrounding tissue. As robotic guidance and imaging advance, this laser tech could transform how surgeons tackle brain tumors.
🫁 Tumors get body doubles. Berlin researchers grew "tumoroids" from lung cancer tissue removed during surgery. These tiny tumor replicas preserve the same genetic and cellular traits as the original, allowing doctors to test different treatments before trying them on patients. When the team tested CAR-T cell therapy, they discovered that a tumor's own defense mechanisms heavily influence whether the treatment works. Within three months of surgery, doctors could use this approach to select the best therapy for each patient.
🧠 Grain-sized mind reader. Cornell University engineers built what may be the world's smallest wireless neural implant - so tiny it can rest on a grain of salt. The MOTE (microscale optoelectronic tetherless electrode) is just 300 microns long and 70 microns wide, using light to power itself and beam brain signals back. After a full year in mice, it reliably recorded neuron activity while barely bothering the surrounding tissue. MRI-compatible brain monitoring could be next.
Cool tool
😴 AutoSleep is a sleep tracking app for Apple Watch that requires zero manual input - just wear your Watch to bed and wake up to detailed insights. Developed by Tantsissa, the app analyzes Apple HealthKit data including motion, heart rate, and sleep stages to measure sleep duration, quality, and deep/REM phases.
The business model is simple: a one-time purchase with no subscriptions, ads, or data uploads. Visual insights appear through colorful clock-face rings showing sleep time, movement, and heart rate patterns, plus readiness scores and sleep bank tracking.
A companion app called AutoSnore uses machine learning to record and classify sleep sounds from your iPhone, including snoring, sleep talking, and coughing. The data syncs with AutoSleep to show how these sounds affect sleep quality.
Weird and wonderful
🤖 AI ate my lab work. University of Cologne plant sciences professor Marcel Bucher just learned tech's hardest lesson: always back up your work.
In a Nature column, Bucher admitted he lost two years of "carefully structured academic work" – grant applications, publication revisions, lectures, exams – after toggling off ChatGPT's data consent option just to see what would happen. Everything vanished instantly. "No warning appeared," he wrote. "There was no undo option. Just a blank page."
Social media responded with predictable schadenfreude, questioning how an academic went 24 months without saving anything locally. OpenAI helpfully recommended that "users maintain personal backups for professional work." Most of us learned that back in 1995.

Image source: Canva AI
Thank you for reading the Healthy Innovations newsletter!
Keep an eye out for next week’s issue, where I will highlight the healthcare innovations you need to know about.
Have a great week!
Alison ✨
P.S. If you enjoyed reading the Healthy Innovations newsletter, please subscribe so I know the content is valuable to you!