Welcome back to Healthy Innovations! 👋
What if scientists could design new medicines as easily as writing computer code?
Breakthroughs in AI protein design, particularly with tools like AlphaFold, now allow us to predict and engineer protein structures with unprecedented accuracy. This advancement enables the creation of targeted therapies for previously untreatable diseases. In just a few years, scientists may be designing custom proteins to treat conditions ranging from cancer to rare genetic disorders.
The protein-engineering market is expected to grow from $3.6 billion in 2025 to nearly $14 billion by 2034 - a fourfold increase. As this technology matures, we're set to see remarkable innovations in healthcare.
So, let's dive in!
How AI is rewriting the code of life
Proteins are the fundamental building blocks of life, performing countless essential functions in living organisms. They build our muscles, carry oxygen in our blood, fight infections, and catalyze the chemical reactions that keep us alive. Yet for decades, scientists struggled with one of biology's most confounding mysteries: how these molecular machines actually work.
The puzzle lies in protein folding.
Each protein starts as a linear chain of amino acids that must fold into a precise 3D shape to function. Get the shape wrong, and the protein fails - sometimes catastrophically, as seen in diseases like Alzheimer's and Parkinson's, where misfolded proteins wreak havoc.
The complexity is staggering.
Even a small protein could theoretically fold into more configurations than there are atoms in the universe. Traditional computational predictions were virtually impossible, making the "protein folding problem" one of biology's greatest challenges.
Until AI revolutionized the field.
In 2020, Google DeepMind cracked this fifty-year-old puzzle with AlphaFold, predicting how proteins fold into their complex 3D shapes. This breakthrough didn't just solve a scientific mystery - it launched a revolution that's transforming how we discover drugs, treat diseases, and understand life itself.
The numbers tell the story: 250 million protein structures now sit in the AlphaFold database, used by nearly 2 million researchers across 190 countries. More scientists can now access protein structures than ever learned to do X-ray crystallography - the painstaking experimental method that previously took years to solve what AI now does in minutes.
This transformation earned Demis Hassabis and John Jumper the 2024 Nobel Prize in Chemistry. But their achievement represents something bigger than academic recognition - it's ushering in what Samuel Hume, a fellow at The Foulkes Foundation, calls the 3rd generation of drug discovery.
1st generation (Nature): Aspirin discovered in willow tree bark
2nd generation (Biotech): Engineered drugs like Ozempic
3rd generation (AI): Able to design proteins from scratch
How AlphaFold works (explained using LEGO!)
AlphaFold 3 is like a super-smart LEGO builder that figures out how tiny pieces in your body fit together. These pieces, proteins, DNA, and other molecules, need to snap into the right shapes to work properly, like building a spaceship from LEGO instructions.
Starting with chaos: AlphaFold 3 begins with what looks like a jumbled cloud of atoms, like dumping thousands of LEGO blocks on the floor with no instructions.
The diffusion magic: The system uses a "diffusion" process, similar to watching a time-lapse video of someone solving a complex 3D puzzle in reverse. Imagine starting with a perfectly built LEGO castle, then adding noise and chaos until it becomes a random pile of blocks. AlphaFold 3 learns to run this process backward - starting with the chaos and gradually removing the "noise" until the correct molecular structure emerges, piece by piece.
Learning from millions of examples: Like a master LEGO builder who has studied every instruction manual ever made, AlphaFold 3 has analyzed millions of known molecular structures. But it goes beyond just memorizing patterns - it understands the fundamental rules of how different molecular "blocks" prefer to connect. It knows that certain amino acids love to stick together, how DNA strands twist and pair, and how drug molecules nestle into protein pockets.
Predicting complex interactions: What makes AlphaFold 3 revolutionary is its ability to predict not just single structures, but how multiple molecules interact, like figuring out how different LEGO sets connect to build a massive space station. It can predict how a potential drug (one LEGO set) will dock with a protein target (another set), or how DNA instructions interact with the protein machinery that reads them.
Speed and accuracy: While traditional methods might take months to experimentally determine how molecules fit together, AlphaFold 3 can generate these predictions in hours with remarkable accuracy. It's like having a LEGO master who can look at loose blocks and instantly visualize the finished creation.
Breakthrough science unlocks new possibilities
AlphaFold has already enabled discoveries that seemed impossible just years ago:
Solving biological mysteries: Scientists cracked the structure of the nuclear pore complex - the "gatekeeper" controlling access to our DNA. This decades-old puzzle was one of biology's biggest unsolved structures, and understanding it opens new research into cancer, aging, and neurodegeneration.
Accelerating drug discovery: Researchers used AlphaFold's serotonin receptor structure to computationally test 1.6 billion molecules, identifying compounds that bind more tightly than any conventionally-discovered drugs. This mental health breakthrough took months instead of years.
Engineering molecular tools: Teams designed a "molecular syringe" that delivers therapeutic proteins directly into human cells - creating entirely new ways to treat diseases at the cellular level.
Real hope for patients
These scientific advances translate into concrete benefits for people facing serious health challenges:
Rare disease: For 400 million people worldwide living with rare diseases, AI offers hope where traditional economics failed. Custom proteins could replace missing or faulty enzymes that cause genetic disorders - conditions that most pharmaceutical companies previously deprioritized due to small patient populations.
Cancer: Instead of chemotherapy that damages healthy tissue alongside tumors, patients could receive treatments that target their specific cancer markers with surgical precision. Early laboratory studies have already identified a liver cancer drug targeting the CDK20 protein.
Autoimmune conditions: Rather than broadly suppressing the entire immune system, patients could benefit from precision interventions that fine-tune immune responses - reducing side effects while maintaining protection against infections.
The ultimate promise: truly personalized medicine where treatments are designed not just for diseases, but for individual patients' genetic profiles.
From lab to clinic
Companies are racing to translate these discoveries into treatments patients can actually receive:
Generate:Biomedicines has moved its first AI-designed antibody into clinical trials, with multiple programs entering human studies over the next two years, proving that AI-designed drugs can meet safety standards for human testing. In 2024, the company also signed a $1 billion deal with Novartis to use its generative AI platform to discover and develop protein therapeutics for multiple disease areas.
Cradle Bio combines AI with robotic laboratories, testing hundreds of AI-designed proteins weekly and compressing optimization cycles from months to days.
Isomorphic Labs have signed pharmaceutical partnerships worth up to $3 billion with Novartis and Lilly, bringing Silicon Valley speed to traditional drug development.
Challenges remain
While AI protein design is making exciting progress, there are still some interesting challenges to work through.
Since AI-designed drugs haven't been tested in humans yet, we're still learning about how effective they might be. Even though lab results look promising, we need to be careful about assuming they'll work the same way in human bodies.
Another challenge is manufacturing - while AI can come up with new protein designs very quickly, actually making and testing these proteins takes much more time, which affects how quickly we can develop new treatments.
These challenges don't take away from how amazing AI protein design is - they just remind us to be patient as we carefully move from laboratory discoveries to real-world treatments.
The programmable future
We're witnessing something unprecedented: the convergence of digital and biological worlds. AlphaFold proves that atoms respond to prompts, that molecular behavior follows predictable patterns AI can master.
This isn't just about making existing processes faster. We're fundamentally reimagining what's possible when we can design biology with the same precision we use to write code.
The protein revolution has begun. And like all great technological shifts, its ultimate impact will likely exceed our wildest current predictions.
Innovation highlights
🩹 Breakthrough bandage tech. Scientists have developed a remarkable "smart bandage" that's could transform wound treatment. It uses innovative electrospun polymer fibers containing antibiotics and is able to deliver targeted medication directly to infections over several hours. Instead of flooding the entire body with drugs, these 2x2cm dressings provide precise treatment exactly where it's needed. This breakthrough technology could revolutionize how we combat bacterial infections and pave the way for a new generation of highly targeted medical treatments.
🩸 Game-changing clot spinner. Doctors have created an incredible "milli-spinner" device designed to save the lifes of stroke patients. This innovative tool uses a spinning tube with fins and slits to precisely shrink and remove brain clots, rather than roughly rupturing them like current methods. The results are remarkable - doubling success rates for most cases and achieving 90% effectiveness on the toughest clots compared to just 11% with existing technology.
Company to watch
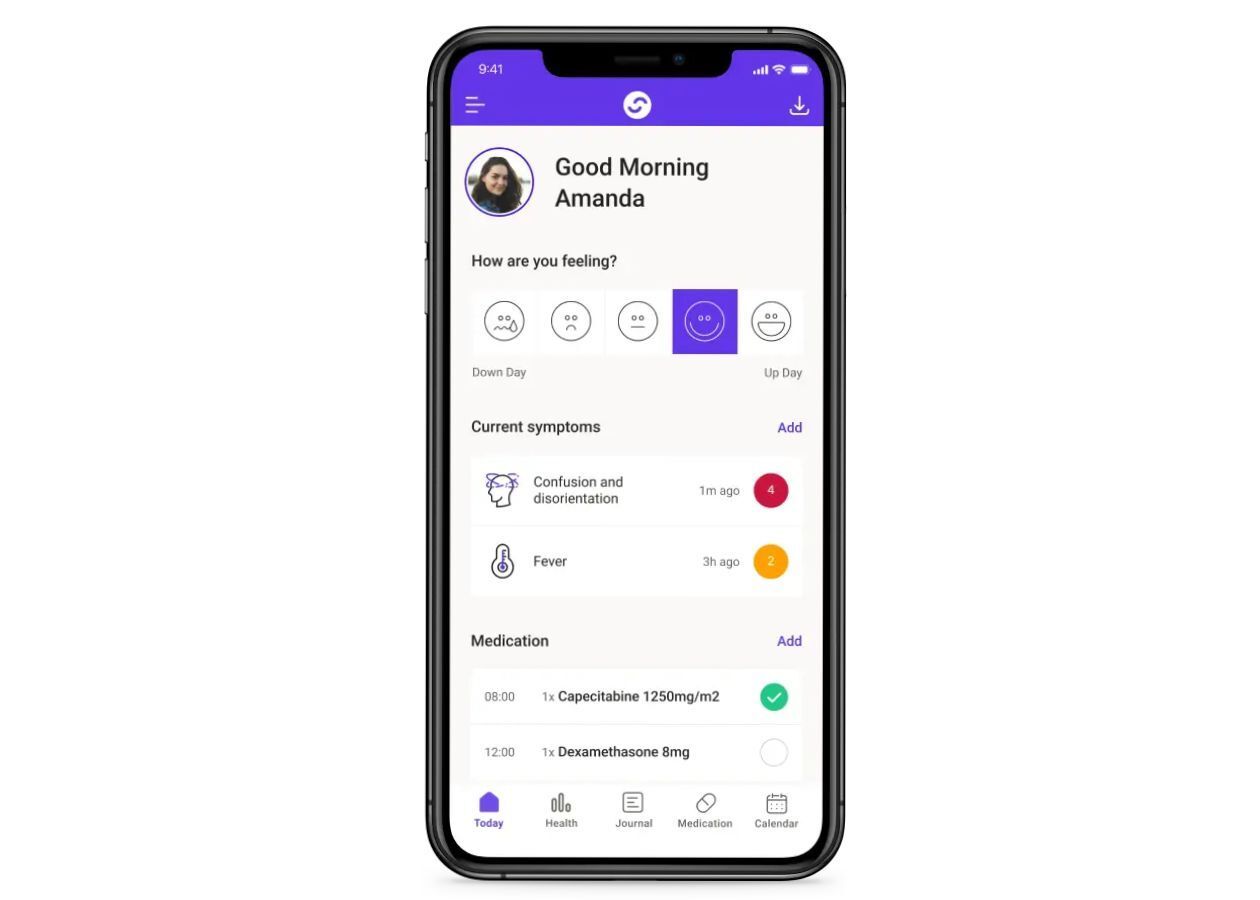
Careology is a UK-based health tech company that’s on a mission to make life easier for people living with cancer.
With Careology, patients can track their symptoms, medication, and appointments using an easy-to-use app. Family members can also stay in the loop and offer support. For doctors and nurses, there’s a professional dashboard that lets them keep an eye on patients remotely, helping to spot any issues early and provide timely care.
Careology is trusted by leading players across the UK and US healthcare sectors, and is being used by several NHS hospitals.
Weird and wonderful
🚭 Unexpected brain boost: Researchers have discovered an intriguing off-label use for nicotine patches and gums – improving cognitive function. Studies show these smoking cessation aids can help with brain fog, ADHD, and even Alzheimer's-related memory issues through controlled nicotine delivery that stimulates attention-related brain receptors.
One writer described feeling like her "brain had come back online" after trying low-dose nicotine gum for concentration issues. While more research is needed, it's fascinating how a product designed to break one habit might accidentally help enhance mental clarity and focus.
Thank you for reading the Healthy Innovations newsletter!
Keep an eye out for next week’s issue, where I will highlight the healthcare innovations you need to know about.
Have a great week!
Alison ✨
P.S. If you enjoyed reading the Healthy Innovations newsletter, please subscribe so I know the content is valuable to you!